Platelet concentrates for surgical use are innovative tools of regenerative medicine, and were widely tested in oral and maxillofacial surgery. Unfortunately, the literature on the topic is contradictory and the published data are difficult to sort and interpret. In bone graft, implant and reconstructive surgery, the literature is particularly dense about the use of the various forms of Platelet-Rich Plasma (PRP) – Pure Platelet-Rich Plasma (P-PRP) or Leukocyte- and Platelet-Rich Plasma (L-PRP) – but still limited about Platelet-Rich Fibrin (PRF) subfamilies.
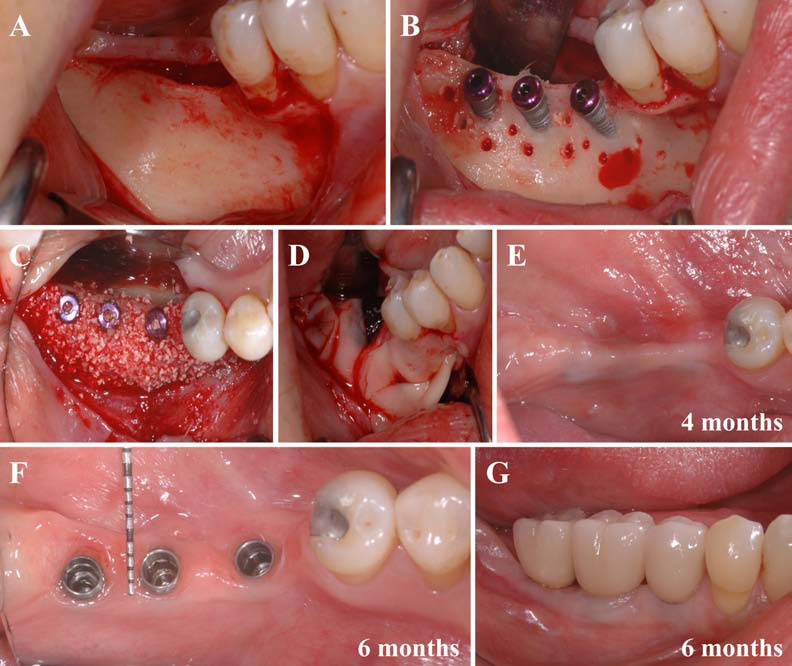
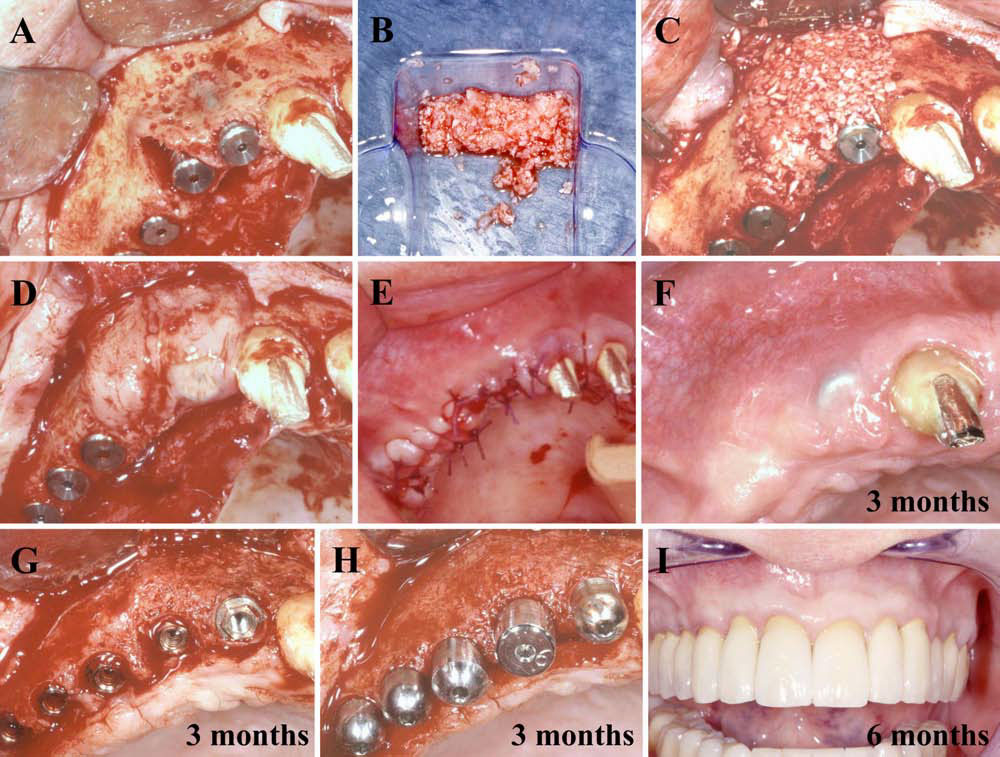
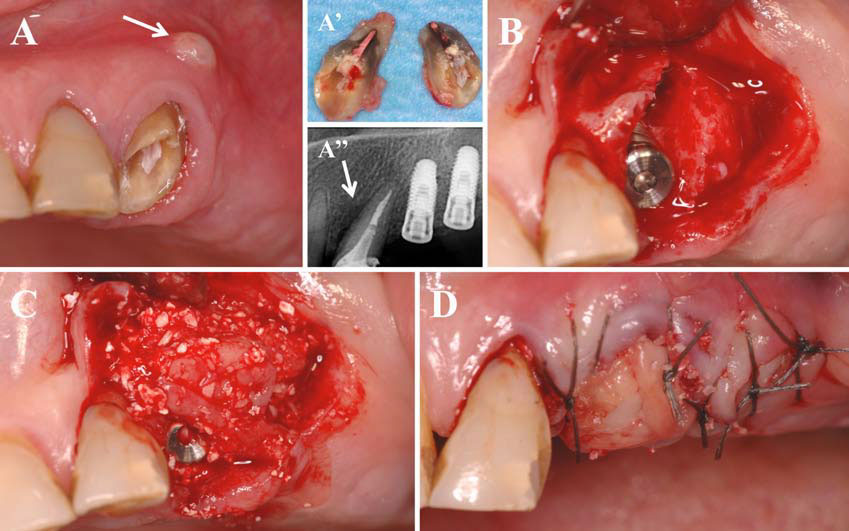
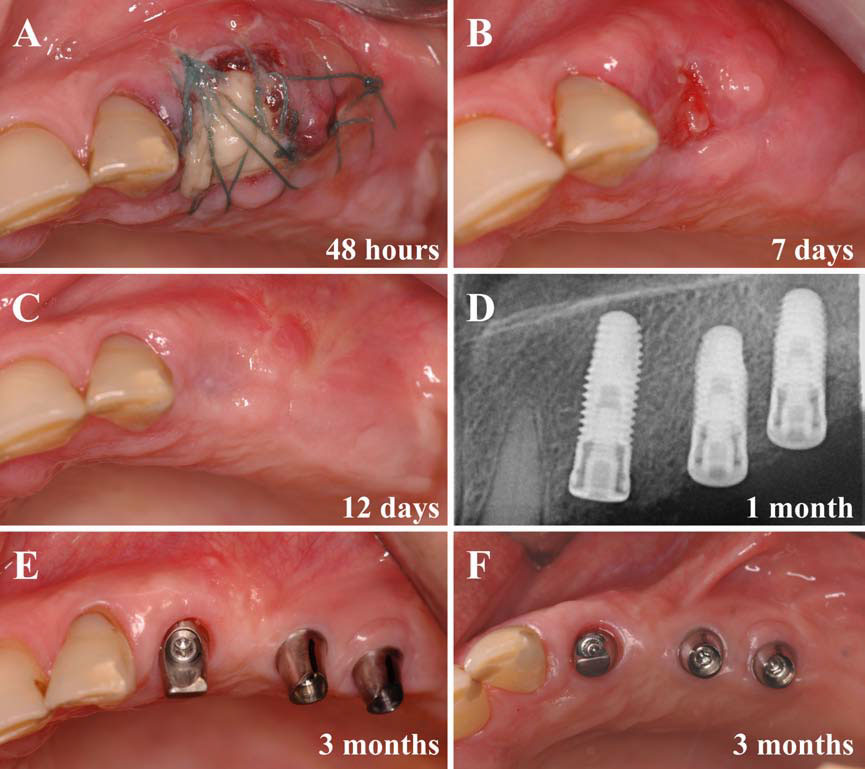
In this second article, we describe and discuss the current published knowledge about the use of PRP and PRF during implant placement (particularly as surface treatment for the stimulation of osseointegration), the treatment of peri-implant bone defects (after peri-implantitis, during implantation in an insufficient bone volume or during immediate post-extraction or post-avulsion implantation), the sinuslift procedures and various complex implant-supported treatments.
Other potential applications of the platelet concentrates are also highlighted in maxillofacial reconstructive surgery, for the treatment of patients using bisphosphonates, anticoagulants or with post-tumoral irradiated maxilla.
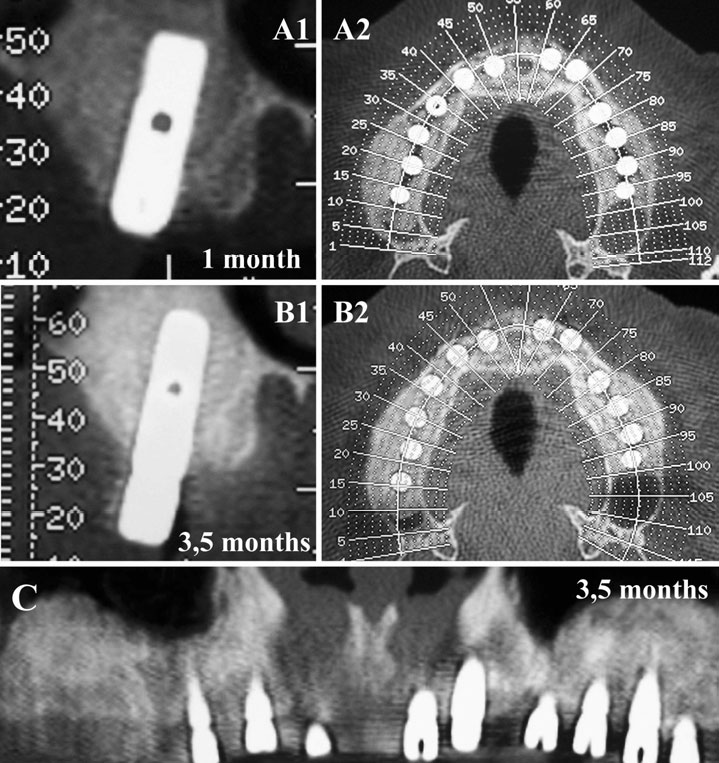
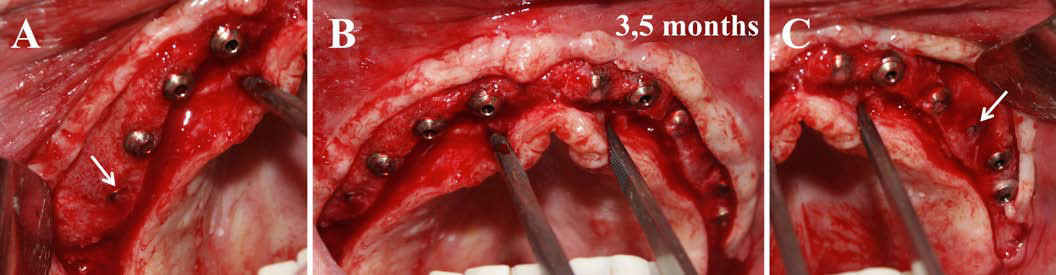
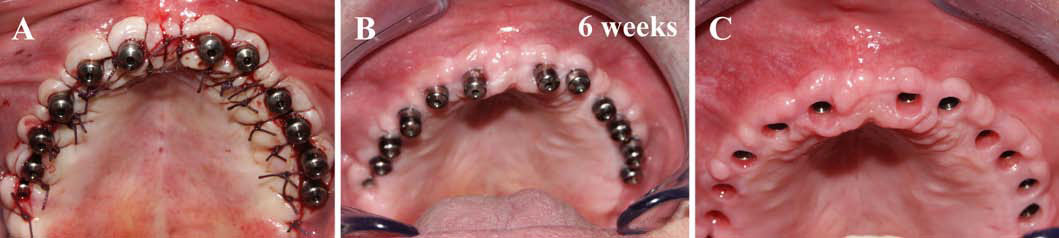
Finally, we particularly insist on the perspectives in this field, through the description and illustration of the use of L-PRF (Leukocyte- and Platelet-Rich Fibrin) clots and membranes during the regeneration of peri-implant bone defects, during the sinus-lift procedure and during complex implant-supported rehabilitations. The use of L-PRF allowed to define a new therapeutic concept called the Natural Bone Regeneration (NBR) for the reconstruction of the alveolar ridges at the gingival and bone levels.
As it is illustrated in this article, the NBR principles allow to push away some technical limits of global implant-supported rehabilitations, particularly when combined with other powerful biotechnological tools: metronidazole solution, adequate bone substitutes and improved implant designs and surfaces (for example here AstraTech Osseospeed or Intra-Lock Ossean implants).
As a general conclusion, we are currently living a transition period in the use of PRP and PRF in oral and maxillofacial surgery.
PRPs failed to prove strong strategic advantages that could justify their use in daily practice, and the use of most PRP techniques will probably be limited to some very specific applications where satisfactory results have been reached.
Only a few simple, inexpensive and efficient techniques such as the L-PRF will continue to develop in oral and maxillofacial surgery in the next years. This natural evolution illustrates that clinical sciences need concrete and practical solutions, and not hypothetical benefits. The history of platelet concentrates in oral and maxillofacial surgery finally demonstrates also how the techniques evolve and sometimes promote the definition of new therapeutical concepts and clinical protocols in the today’s era of regenerative medicine.
AUTHORS
Alain Simonpieri, Marco Del Corso, Alain Vervelle1, Ryo Jimbo, Francesco Inchingolo, Gilberto Sammartino and David M. Dohan Ehrenfest
THIS IS ONLY ABSTRACT. THE FULL ARTICLE IS AVAILABLE DOWN