Platelet concentrates for surgical use are tools of regenerative medicine designed for the local release of platelet growth factors into a surgical or wounded site, in order to stimulate tissue healing or regeneration. Leukocyte content and fibrin architecture are 2 key characteristics of all platelet concentrates and allow to classify these technologies in 4 families, but very little is known about the impact of these 2 parameters on the intrinsic biology of these products. In this demonstration, we highlight some outstanding differences in the growth factor and matrix protein release between 2 families of platelet concentrate: Pure Platelet-Rich Plasma (P-PRP, here the Anitua’s PRGF – Preparation Rich in Growth Factors – technique) and Leukocyte- and Platelet-Rich Fibrin (L-PRF, here the Choukroun’s method). These 2 families are the extreme opposites in terms of fibrin architecture and leukocyte content. The slow release of 3 key growth factors (Transforming Growth Factor 1 (TGF 1), Platelet-Derived Growth Factor AB (PDGF-AB) and Vascular Endothelial Growth Factor (VEGF)) and matrix proteins (fibronectin, vitronectin and thrombospondin-1) from the L-PRF and P-PRP gel membranes in culture medium is described and discussed. During 7 days, the L-PRF membranes slowly release significantly larger amounts of all these molecules than the P-PRP gel membranes, and the 2 products display different release patterns. In both platelet concentrates, vitronectin is the sole molecule to be released almost completely after only 4 hours, suggesting that this molecule is not trapped in the fibrin matrix and not produced by the leukocytes. Moreover the P-PRP gel membranes completely dissolve in the culture medium after less than 5 days only, while the L-PRF membranes are still intact after 7 days. This simple demonstration shows that the polymerization and final architecture of the fibrin matrix considerably influence the strength and the growth factor trapping/release potential of the membrane. It also suggests that the leukocyte populations have a strong influence on the release of some growth factors, particularly TGF1. Finally, the various platelet concentrates present very different biological characteristics, and an accurate definition and characterization of the different families of product is a key issue for a better understanding and comparison of the reported clinical effects of these surgical adjuvants.
1. FOUR DIFFERENT FAMILIES OF PLATELET CONCENTRATES, FOUR DIFFERENT BIOLOGICAL MECHANISMS?
Since the first articles about platelet concentrate tech- nologies that launched the craze for local application of growth factors [1, 2], many authors tried to compare the characteristics of the various PRP (Platelet-Rich Plasma) techniques available. Indeed, more than 10 different proto- cols were marketed, and even more in-house protocols were
proposed, with various centrifugation and separation proce- dures, anticoagulant or activators [3].
Many authors tried first to assess the platelet collection efficiency of the various available techniques and the growth factor content of the various products, but they failed to prove a logical correlation between these 2 parameters [4-
10]. Three key platelet growth factors were particularly in-
vestigated: Transforming Growth Factors 1 (TGF 1), Plate- let-Derived Growth Factors AB (PDGF-AB) and Vascular Endothelial Growth Factors (VEGF). Unfortunately, the lit- erature on growth factor release in PRPs is not very relevant, since most growth factor quantifications were performed on the platelet suspensions before activation, and were therefore meaningless: the true growth factor content could only be assessed after full activation of the product. It was also sus- pected that many reported data were not using the right scales [11], reporting nanograms/mL (and sometimes micro- grams/mL)[5] of TGF 1 and PDGF-AB, when the normal concentrations for unactivated products should obviously be in picograms/mL.
But the main problem was in fact much deeper: a lack of pertinent terminology and classification led to severe confu- sions between the many available products and to inadequate methodologies of analysis [12-16]. Most tested products were not fully characterized, and important data such as the leukocyte content [17] and fibrin architecture [18-21] were not assessed [22, 23]. The formation of the fibrin matrix gel during platelet concentrate activation [24] and the leukocyte concentration and formula are obviously key parameters of the growth factor release [11, 25, 26], and also present a strong biological impact in the healing equation [17, 18, 27-
29], particularly against infections [30-32]. Without the con- trol of these parameters, the contradictory literature about the effects of the platelet concentrates was mostly focused on the platelet count, and was therefore often biased.
Recently, a full classification of platelet concentrate technologies was designed [3], and allowed to classify the main available techniques in 4 families, depending on their leukocyte content and fibrin architecture:
• Pure Platelet-Rich Plasma (P-PRP) and Leukocyte- and Platelet-Rich Plasma (L-PRP) are liquid platelet suspen- sions, respectively without and with leukocytes. They can be used as injectable suspension, particularly in sports medicine [33, 34]. After activation (with throm- bin, calcium chloride, batroxobin or others agents), these preparations become respectively P-PRP and L-PRP fi- brin gels, with a brutal and incomplete fibrinogen po- lymerization and a light final fibrin architecture.
• Pure Platelet-Rich Fibrin (P-PRF) and Leukocyte- and Platelet-Rich Fibrin (L-PRF) are solid fibrin biomateri- als, respectively without and with leukocytes. In these techniques, the platelet activation is part of the produc- tion process: it can be natural (L-PRF) or artificial (P- PRF) but always occurs during the centrifugation, and leads to a strong final fibrin architecture.
The concept of this classification is to define and regroup the products through their main features and associated bio- logical mechanisms. This is only a first step, since platelet concentrations, leukocyte concentration and formula can also vary within a family of products. This classification is very attractive and logical from a strict theoretical standpoint, but it is now necessary to highlight and clarify the different bio- logical properties and mechanisms associated to each family.
2. EVALUATION OF THE IMPACT OF THE FIBRIN MATRIX AND THE LEUKOCYTE CONTENT ON THE GROWTH FACTOR RELEASE.
2.1. Definition of Anitua’s PRGF (P-PRP) and Chou- kroun’s PRF (L-PRF)
In order to understand the different biological mecha- nisms of the 4 families of platelet concentrates, the first step is to compare the growth factor release of well-characterized products from 2 different families. Two products seemed the adequate examples.
The first tested product is a P-PRP called PRGF, aka Plasma [35] or Preparation [36] Rich in Growth Factors. This manual procedure was invented by Anitua in 1999 [35] and is currently marketed by BTI, aka BioTechnology Insti- tute (Vitoria, Spain), a dental implant company directed by Dr Anitua [13]. After a soft centrifugation of blood with an- ticoagulant, four layers appear in the tube: the superficial plasma suspension was often called PPGF (Plasma Poor in Growth Factors), the intermediate plasma suspension is called PRGF (Plasma Rich in Growth Factors), the whitish layer below the PRGF is called the « buffy coat », and the red blood cells are finally gathered at the bottom of the tube. The PPGF and the PRGF are collected by pipetting the plasma solution above the red blood cell base. The specific- ity of this technique is that the buffy coat layer (between the red blood cells base and the acellular plasma) is not har- vested in order to avoid the collection of leukocytes [37]. Since the buffy coat layer contains most leukocytes and also many platelets, the final PRGF is a platelet-rich plasma sus- pension with almost no leukocyte and a lower platelet con- centration than other PRPs [38]. The PRGF supension can be used like an injectable pharmaceutical solution, particularly in sports medicine [39] or for the coating of implant surface [40, 41](even if this last application is very debatable)[42], or can be activated with calcium chloride in order to prepare a fibrin gel. This fibrin gel is quite fragile and unstable, and can be used as a fibrin covering layer, like a fibrin glue [37,
43].
The second tested product is a L-PRF, called Choukroun’s PRF. This open-access technique was invented in 2000 by Choukroun [44], and adequate kits and centrifuge were marketed by Process (Nice, France). The general con- cept of this technique is very different from the numerous other protocols and was often considered as a second genera- tion platelet concentrate technology [45-48]. Blood is taken without anticoagulant and immediately softly centrifuged. Blood activation occurs during the centrifugation and allows the formation of a dense fibrin-platelet clot in the middle of the tube, between the red blood cell base at the bottom and the acellular plasma at the top of the tube. The L-PRF clot contains almost all the platelets and more than 50% of the leukocytes from the initial blood harvest, and presents a strong fibrin architecture and a specific three-dimensional distribution of the platelets and leukocytes [16, 49]. This product therefore only exists in an activated form and can not be injected like a suspension. Because of its strong fibrin architecture, this solid biomaterial is particularly useful in oral and maxillofacial surgery [50-53], periodontology [54,
55], implant dentistry [56-62] and ENT (Ear Nose Throat) surgery [63-65], and many other applications may be inves- tigated in the future.
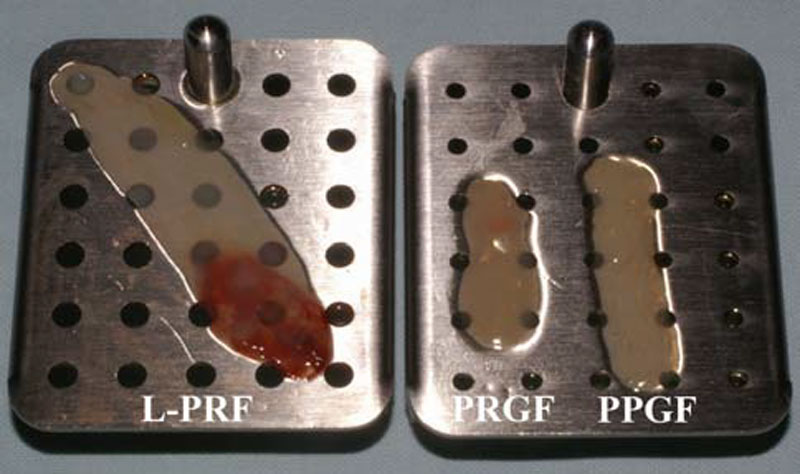
PRGF gel and Choukroun’s PRF are therefore the 2 ex- treme opposites of the platelet concentrate technologies, re- spectively a P-PRP gel and a L-PRF. The 2 materials can easily be prepared in similar solid forms and volumes, and the comparison of their growth factor release is of great in- terest in order to understand the impact of the leukocyte con- tent and the fibrin architecture on the intrinsic biological mechanisms of these living biomaterials.
2.2. Comparison of the Protein Release Patterns
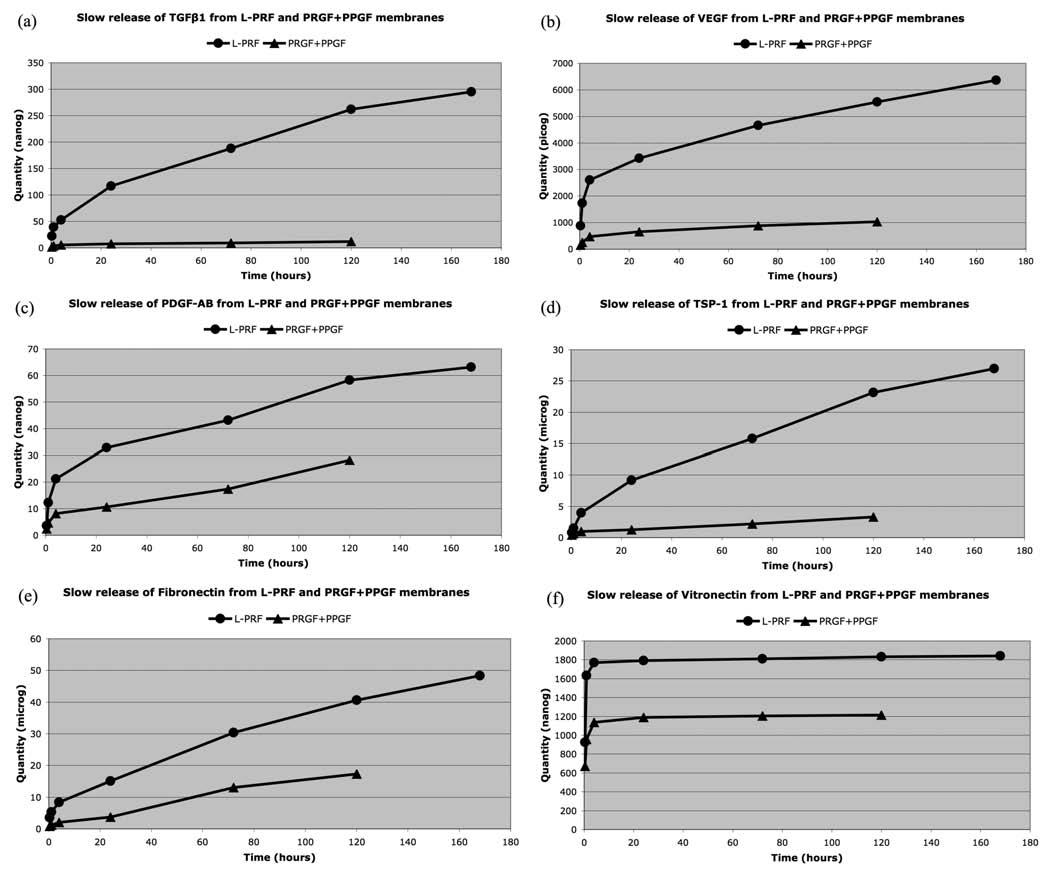
In a recent study, it was proven that a L-PRF membrane slowly releases significant amounts of some growth factors (TGF 1, PDGF-AB, VEGF) and thrombospondin-1 (TSP-1) during at least 7 days [11]. The method was simple: the L- PRF membrane was placed in a 10 mL tube with 4 mL of sterile Dulbecco’s modified eagle’s medium (DMEM). Then, at each experimental time, the membrane was transferred in a new tube with 4 mL sterile DMEM, and the previous 4 mL were stored at -80°C before ELISA quantification. The membrane transfer was done at seven experimental times: 20 min, 1h, 4h, 24h (day 1), 72h (day 3), 120h (day 5) and 168h (day 7). The initial growth factor content of a L-PRF mem- brane after forcible extraction was also quantified. The com- parison between the 7-day slow released quantities and the initial growth factor content highlighted interesting data: the PDGF-AB initial quantities were quite similar to the final released amounts after 7 days. But the released amounts of TGF 1 and VEGF after 7 days were more than 6 times higher than the initial content. It was thus hypothesized that the leukocytes trapped within the L-PRF membranes were producing these growth factors large surplus.
Following exactly the same protocol and using the same four volunteer blood donors than in the first article (two males and two females, non-smokers, aged between 50 and
55 years old), we decided to evaluate the release of the 3 same growth factors (TGF 1, PDGF-AB, VEGF) and 3 key matrix proteins (thrombospondin-1, fibronectin and vi- tronectin) from a L-PRF membrane and from a PRGF gel membrane. Fibronectin and vitronectin are 2 key cell adhe- sion and migration proteins. The fibronectin is present in great quantities in a blood circulating form and in the plate- lets [66], and is also a key component of the architecture of the fibrin clot [67]. Therefore, the fibronectin released from a platelet concentrate gel is first the soluble free fibronectin, and not the fibronectin blocked in the in-depth architecture of the fibrin matrix.
Choukroun’s PRF (Process, Nice, France) and Anitua’s quantifications of growth factors and matrix proteins were performed in triplicate with ELISA kits (Quantikine, R&D Systems, Minneapolis, MN, USA, for TGF 1, PDGF-AB, VEGF and Thrombospondin-1 ; EIA kit, GenWay, San Di- ego, CA, USA, for fibronectin and vitronectin).
The results are reported in the Table 1 and in the Fig. (2), and demonstrate the impact of the cell content and the fibrin architecture in the biology of these platelet concentrates.
First, after 20 minutes the release of growth factors and matrix proteins from the L-PRF is always very significantly (p<0.0001) superior to the release of the PPGF and PRGF membranes together, during the 7 days of experiment.
Second, at the 5th day of the experiment, the PPGF and PRGF membranes were completely dissolved in the culture medium. The 2 membranes looked already damaged on the
PRGF (BTI, Vitoria, Spain) were produced with their official third day, and disappeared between the 3rd and the 5th days of kits and following the classical protocols previously de- scribed in the literature. For the Choukroun’s PRF, blood was harvested in 9mL glass-coated plastic tubes, and the L- PRF clot was directly collected at the end of the centrifuga- tion. For Anitua’s PRGF, blood was collected in two 4.5mL citrated tubes, and after centrifugation, the 2 PPGF were gathered in a tube and the 2 PRGF gathered in another tube for separate clotting by adding calcium chloride and waiting a dense polymerization for 1 h at 37°C. All the membranes were finally prepared and standardized using the PRF Box (Process, Nice, France), an user-friendly device that allows to compress the PRF and PRGF/PPGF clots into membranes in a sterile and protected environment [68]. Finally, with the same 9mL blood volume, we produced one big L-PRF mem- brane and 2 small P-PRP gel membranes (one PRGF and one PPGF). The PRGF and PPGF membranes together presented almost the same volume than a L-PRF membrane alone Fig. (1). The comparison of the growth factor releases from the L-PRF membrane and the PPGF/PRGF gel membranes is therefore particularly relevant. Three L-PRF and three PPGF/PRGF membranes were produced for each donor. The the experiment. On the contrary, the L-PRF membrane seemed intact after 7 days in culture, as it was reported previously in various in vitro culture studies [69, 70], and the L- PRF could probably remain in the experiment even longer. This observation confirmed that the fibrin architecture is much stronger in the PRF subfamilies than in the PRP gel classes.
Third, the growth factor releases of the PPGF membranes were not significantly different from the releases of the PRGF membranes; this result was associated to very large standard deviations of the measured mean quantities of re- leased proteins from the PRGF and PPGF. This observation was quite surprising, since the PPGF was supposed to pre- sent a lower platelet concentration than the PRGF. However, there is a simple explanation: the PRGF technique is a man- ual procedure that requires several steps of pipetting with eye-balling a sole measuring method. The separation of the PPGF and PRGF layers in the plasma supernatant is very theoretical, since the buffy coat is not collected. Pipetting in a small tube always creates turbulences that unavoidably homogenize partially the PPGF and PRGF layers. Our results therefore demonstrate that the PPGF and PRGF layers present similar biological signatures, and that their theoreti- cal separation is an empirical concept.
Finally the release patterns were very different between the L-PRF and the PPGF/PRGF membranes, and give us considerable informations about the effect of the fibrin archi- tecture and the leukocyte content on the mechanisms of the release. The L-PRF membrane slowly released large amounts of TGF 1, PDGF-AB, VEGF, thrombospondin-1 and fibronectin during at least 7 days. If the stronger release occurs during the first 24 hours, the membrane released large amounts continuously during the whole experiment. On the contrary, in PRGF/PPGF membranes, TGF 1, PDGF-AB, VEGF and thrombospondin-1 were mainly released during the first 4 hours and, even if a small release continued up to the final dissolution of the PPGF/PRGF membranes, this slow release was considerably less intense than in a L-PRF membrane (this can be easily observed on the slope of the release curves in the Fig. (2). The situation was even more obvious with the fibronectin, that was released in 2 phases from the PRGF/PPGF membranes: half of the total content was released massively during the first hour, and the remain-
ing fibronectin was released between the 3rd and 5th days when the PPGF/PRGF membranes finally dissolved. The explanation of these different release patterns is quite simple: during the platelet and fibrinogen activation of PPGF/PRGF, growth factors and some other proteins are not enmeshed in the fibrin network, because the fibrin polymerization is in- complete. These molecules are therefore released massively during the first hours after preparation. Moreover, these products do not contain leukocytes and can therefore not sustain the production of new growth factors after the initial release. On the contrary, the strong fibrin architecture of the L-PRF allows an intense slow release during the whole ex- periment, and the release is even supported by the production of new growth factors (particularly TGF 1) by the leuko- cytes living in the L-PRF membrane.
There is only one exception to these release patterns: in L-PRF and PPGF/PRGF membranes, the vitronectin was massively released during the first 4 hours, and the release was then almost nil during the next 7 days. This observation demonstrates that some molecules can not be trapped in the fibrin matrix, whatever the method of polymerization, and are released almost immediately after production. It also means that if we need vitronectin on a surgical site, the membranes should be used quite quickly after preparation (even if the conservation in a PRF Box may probably de- layed the vitronectin release) or we should also use the clot exudate collected in the PRF Box [68].
3. A SIMPLE DEMONSTRATION WITH LARGE PERSPECTIVES.
The release patterns of the 6 tested molecules demon- strate the very significant differences between 2 families of products, P-PRP and L-PRF, and this is therefore an impor- tant step for the definitive validation of the current classifica- tion of platelet concentrate technologies. Even if both prod- ucts are platelet concentrates, their intrinsic structure, biol- ogy and molecular kinetics are completely opposite. The growth factor release was much more intense in the L-PRF than in the PRGF, but it is not possible to claim that one technique would be better than the other: both families of products can have a potential positive impact during healing, and the 2 technologies have simply different indications since P-PRP are injectable liquid platelet suspensions, while L-PRF only exists as a solid dense fibrin biomaterial.
However, our results suggest that when a fibrin gel bio- material is needed, L-PRF clots or membranes should be preferred to the PRGF gel: indeed, the L-PRF releases much more growth factors and key adhesion proteins during a longer time period and presents a much stronger fibrin archi- tecture than the PRGF gel. Moreover, the L-PRF technique is very simple and inexpensive, while the PRGF production and clotting technique requires many handling steps and is very time-consuming in order to produce a usable fibrin membrane (clotting during 1 hour at 37°C !). This conclu- sion contradicts the published opinion [71] of the « PRGF team » of the BTI company who claimed that the PRGF technology could be used in many different forms: in the gel form, PRGF is probably not the most adequate platelet con- centrate to use, particularly in some applications that require a strong fibrin matrix and simple production procedures of many membranes, such as in oral and maxillofacial surgery.
Logically, this conclusion is true for all P-PRP gels, and not only the PRGF, even if there may be substantial differences in the platelet content between the various P-PRP techniques.
This demonstration of the impact of fibrin architecture and leukocyte content on the biological signature of these products is a first step that opens many perspectives. First, since the various families of product seem to use different intrinsic biological mechanisms, it could be very interesting to investigate properly the effects of these different mecha- nisms on the proliferation and differentiation of various cell lineages in vitro: the current literature on this topic is often confusing and contradictory because of the lack of proper characterization of the products and associated methodologi- cal bias [15]. The following step would be to compare the clinical impact of these 2 families of products. These kinds of comparative studies could increase our knowledge about these products, but remain difficult tasks. Second, our dem- onstration militates for the refoundation of the current con- fusing literature about platelet concentrates, by following the simple principles of the classification system [3]. Products should be completely characterized before testing. Fibrin architecture and leukocyte content can not more be neglected in this field.
The perspectives of research are however not only related to platelet concentrates for surgical use. Indeed, blood is a low-cohesion circulating tissue, and it truly gets a full solid tissue cohesion only when it assembles itself in a dense fi- brin network during the coagulation process: investigating the mechanisms of L-PRF and P-PRP is therefore not only a way to understand the platelet concentrates technologies, but also a way to understand the blood biology.
Several in vitro articles already demonstrated that the dense fibrin architecture of a L-PRF membrane allowed a long slow release of many molecules [14, 68, 72, 73], while the membrane itself was still almost intact after 7 days in the culture medium, even in contact with various cell types in culture [15, 69, 70]: the L-PRF membrane behaves like a true fibrin tissue. L-PRF is often considered as an optimized blood clot [49], and it is indeed a very good illustration of the solid form of the circulating tissue. This slow release was not expected for the products from the PRP families: indeed, these products are brutally activated using bovine thrombin, calcium chloride or other clotting agents, and it was not ex- pected that the platelet growth factors could be trapped in- trinsically in this artifical light fibrin network [14, 24, 26,
72]. Our data therefore confirmed the current knowledge about the fibrin clotting [18, 21], and that platelet concen- trates may be very interesting models for a better understand- ing of coagulation and healing mechanisms.
As a conclusion, the key issue in platelet concentrate technologies is not the quantity of platelets, but how platelet, leukocytes, fibrin and growth factors are interlinked in the final product. A strict quantitative approach does not allow to define the biological signature and mechanisms of a fam- ily of platelet concentrates. The approach must be qualita- tive. PRPs and PRFs are not pharmaceutical preparations with a simple and clear composition, they are living tissues which properties are dependent on the combination of cells, factors and matrix within the final preparation. This demon- stration highlights the different mechanisms between 2 families of platelet concentrates, and the significance of the cur- rent classification system in order to clarify the literature on the topic and to define adequate research strategies.
DISCLOSURE OF INTEREST
The authors declare no competing financial interests.
ACKNOWLEDGEMENTS
This work was supported by the LoB5 Foundation for
Research, Paris, France.
AUTHORS
David M. Dohan Ehrenfest,*, Tomasz Bielecki, Allan Mishra, Piero Borzini, Francesco Inchingolo, Gilberto Sammartino, Lars Rasmusson and Peter A. Everts
THE FULL ARTICLE IS AVAILABLE DOWN