|
Maxillary atrophy is an increasingly common clinical condition and its management requires patient-specific procedures, allowing for a reduced intra-operative timing and maximum postoperative compliance. The causes that lead to focal or generalized atrophy lie in multiple factors, but edentulism plays a primary role. After loss of compromised teeth, resorption is maximum in the first year and more marked in the anterior areas than in the posterior ones. In the following years, there is a minimum but constant decrease in the residual bone quantity. Besides, it is the only material having osteogenic properties in addition to osteoinductive and osteoconductive properties. From the second half of the 1990s, the attention of the “Oral and Maxillofacial Surgery Community” was attracted by a series of scientific papers: they claimed that a platelet-derived growth factor could be valid not only for hemostasis, but also in the emerging field of bone grafting. In the last years, a platelet concentrate called PRF (Platelet Rich Fibrin) was tested for the first time in France by Choukroun et al. PRF belongs to a new generation of platelet concentrates: PRF is obtained without adding anticoagulants like heparin, EDTA, bovine thrombin etc. During the production of PRF, other cellular elements like leukocytes are activated, in addition to platelets. After the artificial hemostatic and inflammatory phenomenon induced by centrifugation, they release cytokines. Then we will find three pro-inflammatory cytokines (IL-1â, IL-6 e TNFá), an anti-inflammatory cytokine (IL-4) and a key promoter of angiogenesis (VEGF) PRF is then able to regulate inflammation and to stimulate the immune process of chemotaxis. PRF is an autologous grafting material that eliminates any risk of disease transmission; besides, its jelly-like consistency favors stability of the clot and of the grafting material. This natural material seems to accelerate the physiological wound healing; besides, in association with bone grafts, it seems to accelerate new bone formation. PRF has many advantages: Simple and cheap protocol Contains a great quantity of fibrins, platelets and leukocytes 18 Accelerates angiogenesis 14,15, multiplication of fibroblasts and osteoblasts, and cicatrization. Aim of the work: the aim of the present study is to investigate, clinically and histologically, about the potential of PRF, used as grafting material in pre-implant reconstructive surgery of severe maxillary atrophy; in particular, we want to assess what changes histologically and clinically after sinus lift procedures at 106-120-180 days, to determine if the use of PRF is able to accelerate the process of bone regeneration, which is essential to promote implant stability. This study also includes a control group, in which only deproteinized bovine bone (Bio-Oss) was used as reconstructive material, used without PRF. Materials and Methods Patients were recruited using the cluster-sampling technique, in order to obtain a heterogeneous sample, representing the macro-area of South Italy.
Inclusion criteria were:
The major atrophies in selected patients involved the sinus-lift procedure, with a second-look reopening for the implant insertion phase. Before any treatments, the patients were properly informed about the advantages and disadvantages of all the planned procedures, first verbally and then with a written form for the informed consent. The present study always obeyed the rules established in the 1975 Declaration of Helsinki, then revised in 1983. Preparation for surgery Preparation of PRF The PRF obtained will be used in two ways:
First Surgical Stage Maxillary sinus lift was performed according to Tatum’s technique, involving an incision on the crestal ridge of the maxilla in order to access to the maxillary sinus. The grafting materials were: With the bone widow being partially occluded by the added PRF, we predisposed the insertion of Bio-Oss mixed with a small quantity of gelatinous PRF, in order to have a mixture that could be compacted in the crest area, under the small masses of amorphous PRF that were previously placed. This led to a clear mechanical effect of compression, favoring the compacting of the grafting material in the crest area and, at the same time, reaching the goal of sinus lift. At the end of this stage, two membranes were placed. They were obtained from the flattening of the remaining PRF between two sterile gauzes and then placed in order to close the access area to the sinus site. Lastly, the bone window was closed to avoid traumatisms, the flap was replaced and then the mucosal areas were sutured through a non-resorbable material. Second Surgical Stage The surgical phase of sinus lift, performed in two stages, as the protocol requires, did not allow proceeding with implant surgery due to the small quantity of maxillary bone (< 5mm). The reopening of the surgical area was scheduled at different times. The 12 patients who received bilateral lift allowed to evaluate the result of both PRF technique and Bio-Oss only technique. Therefore, in these patients, there will be both the “Test-Side” and the “Control-Side”.
4 patients presented with a major bilateral atrophy, therefore received two sinus lifts with the use of PRF and Bio-Oss (Test-Side) and of Bio-Oss only (Control-Side). The sampled material was sent to a lab of Anatomical Pathology. Table 1 Synoptic evaluation of patients.
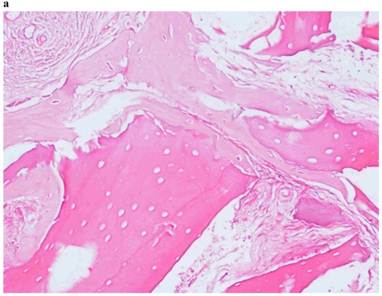
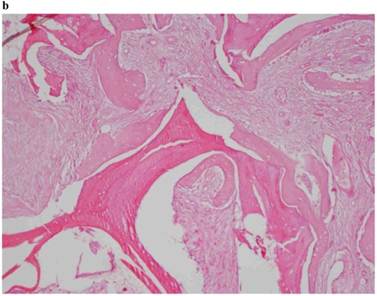
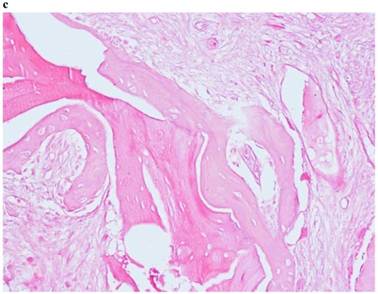
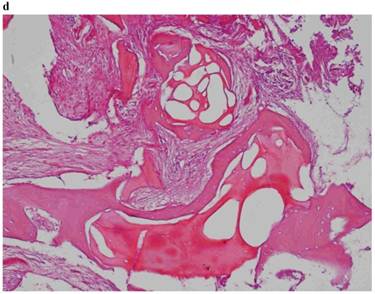
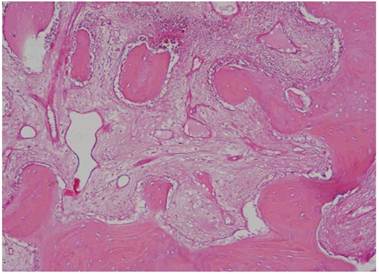
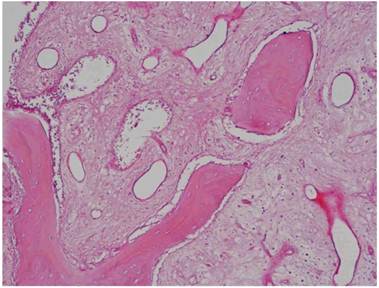
Histological Analysis Results Histological Results
Histomorphometric Results However, the histomorphometric investigation and the histological analysis revealed that the good osteoconductive capacity of PRF leads to the production of new bone, already 106 days after reconstructive surgery. It was impossible to verify the last Albrektsson’s implant success criterion 1 (maintaining of implants on 85% at 5 years, on 80 % at 10 years), because the patients had been followed for less than 3 years. Primary implant stability was assessed by means of RFA (Resonance Frequency Analysis) (Osstell®). Data of measurements was recorded as ISQ values (Implant Stability Quotient): for each implant were performed three measurements, and from these values, a mean value was calculated and recorded. Mean ISQ value was 37.2 (sd: 4.2) in “early protocol group” implants, 36.8 (sd:6.1) in “intermediate protocol group” implants and was 39.1 (sd: 9.0) in “late protocol group” implants. The real increase in perimplant bone density was evaluated by the analysis of Orthopantomographies and Dentascan x-rays, as well as by a personal computer and by the “Implant3D” software, which allowed for a three-dimensional reconstruction of the treated area. The tomographic sections were scanned and analyzed by computer software, so that the tomograms corresponding to the pre- e post-operative conditions could be compared. Table 2 Histomorphometric Evaluations. We evaluate the results of “Test-Side” and “Control-Side” in the three protocols.
Discussion The purpose of treating toothless areas with endosseous implants has often clashed with the assumption that the loss of teeth is reflected in a progressive bone resorption 25. Platelet Rich Fibrin (P.R.F.) was first described by Dr. Choukroun and introduced with the European Directive n. 2004/23/CE of March 31, 2004. Histological studies showed an equal bone growth and trabecular organization between the areas treated with PRF and those of the control sample (F.D.B.A.); the rate of vital bone/inert bone of the neoformed trabecular bone revealed that, in these studies, about 1/3 of neoformed bone graft is inert while over 2/3 of new bone is vital. The Authors concluded that, with the aid of PRF, the healing time is significantly reduced and the implant can be placed already 4 months (120 days) after surgery. A histological control 4 months later revealed that bone quality between the areas treated with PRF and FDBA and the control areas were the same 8. Conclusions Platelet-rich fibrin is a grafting material that eliminates any risk of xenopathy transmission; besides, its gelatinous consistency favors clot stability and the membranous shape allows creating a natural “barrier effect” on the bone breaches that were opened in the surgical areas. Acknowledgements AUTHORS 1. Albrektsson T, Zarb GA, Worthington P. et al. The long-term efficacy of currently used dental implants: a review and proposed criteria of success. Int J Oral Maxillofac Impl. 1986;1:1-25 |